|
BRIEFING - November 2000
A smarter way to buy
As demand for vaccines outstrips supply, Lisa Jacobs finds out how the system for buying them is modernizing
IF only it were as easy as telling a company, "We need three times more vaccine this year than we did last year. If you can make it, we will buy it." Crank up the manufacturing capacity and start producing, right? Not quite. A company must invest huge amounts of capital and time to build a new vaccine production unit, or create a significant expansion. So when vaccine needs change over time, supply can be hard to find.
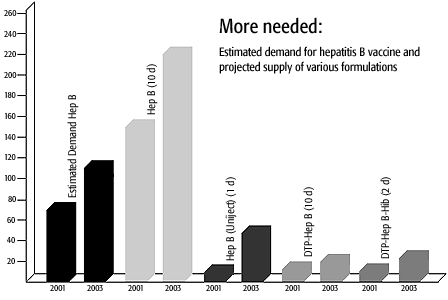 |
The emergence of GAVI and the Vaccine Fund has generated a great deal of new demand for hepatitis B (hep B) vaccine. And already, GAVI has run into a problem with supply. While global capacity for supply of monovalent hep B vaccine is sufficient to meet demand, a significant proportion of the demand is for combined diphtheria, tetanus and pertussis (DTP) and hep B. And while a number of companies are developing this combination vaccine, only one company, SmithKline Biologicals, currently has this combination pre-qualified by WHO and available for sale. Significant increases in supply will not be seen very quickly.
In the meantime, GAVI Board has made a policy decision to reserve the available stock of combination vaccines for countries with relatively weaker systems. The burden of introducing new vaccines – additional training, cold chain and logistics requirements – are minimized through the use of combination vaccines. Furthermore, vaccines given in combination necessitate fewer injections per child, thereby enhancing safety. The countries with the stronger systems will be encouraged to introduce the monovalent vaccine into routine immunization.
"The situation is not ideal, but until combinations become available in greater quantity, we have tried to develop a transparent and equitable allocation policy that ensures the highest degree of safety and allows as many countries as possible to access combination vaccines through the Vaccine Fund," says Steve Landry, of USAID and a member of the GAVI Working Group.
At press time, officials from UNICEF and WHO were to meet with a number of developing country health officials to discuss the available vaccines, and try to match need with supply.
A predictable supply
Vaccines are unlike most products – pharmaceutical and otherwise – in that they are biological compounds. They are alive, needing cultivation and care through their entire development process, sensitive to tiny discrepancies in manufacturing procedures.
All components for their manufacture must be constructed to very specific standards of temperature, moisture, air pressure and of course, safety and hygiene. Not only is scaling up capacity time-consuming, vaccine production is also highly regulated with each batch, or lot, requiring stringent
testing. When errors occur – and they do occur – a whole lot can be rendered useless.
The size of investment necessary to create and maintain vaccine manufacturing capacity, therefore, means that companies must operate at or near full capacity, most of the time. Sudden increases in demand cannot be easily met. Scaling up can take years.
This is all to say that the manufacture of vaccines is fraught with difficulty and risk. If a manufacturer underestimates demand, it risks losing market share; if demand is over-estimated, expensive facilities are underused, and investments turn into losses.
Buying vaccines for the world’s children
The six vaccines introduced on a large scale in the developing world through the global Expanded Programme on Immunization, or EPI, were already mature products when the programme started. Product maturity in the vaccine field means that vaccine production is smoother, more manufacturers in the field means that demand can be more easily met, and more manufacturing capacity means that costs are reduced, and prices can follow costs down.
In this environment, it made sense for UNICEF to pursue a procurement policy that achieved the lowest prices possible. To do this, it has used what is known as a tender approach – issuing requests to all qualified manufacturers every or every other year for quotes on a specific amount of a particular vaccine.
This policy has its benefits; the low vaccine prices achieved through this method were critical in helping even the poorest countries introduce basic routine immunization into their health systems. But the singular focus on price – with the necessary attention to quality, of course – has downsides as well. For one thing, since EPI began in the 1970s with six vaccines, new vaccines have been
developed. Many children in the richer countries are now protected against 11 or 12 vaccine-preventable diseases. But the newer vaccines are not available for most children in developing countries, for two reasons that are inextricably linked: greater costs and limited supply.
Developing country health programmes have come to regard vaccines as low price commodities. In fact, vaccine costs are only a small fraction of the total estimated cost of providing routine immunization to a child. Of the estimated $20 it costs to protect one child, the cost of the required doses of the six vaccines comes to less than $1 – the great majority of costs involved are staff, vehicles and maintenance, cold chain equipment, training and other overhead. The thought of paying more than a few pennies for the vaccine is anathema to many health officials.
But the focus on low prices has not helped to convince manufacturers that making the necessary investments on development and manufacturing capacity for vaccines for the developing world can make business sense.
A new approach to procurement
With the emergence of GAVI, and its focus on reducing the gap between the time that a vaccine becomes available in rich and poor countries, UNICEF Supply Division has taken the opportunity to restructure its relationships with companies. For the purchase of new and under-used vaccines, UNICEF has decided to use a ’request for proposals’ method.
Through this route, UNICEF is asking companies to make offers that cover a longer term, affording companies more stable commitments over three to five years to ensure sustained supply of vaccines over longer periods. In addition, the vaccine industry has offered UNICEF additional components to contribute to the GAVI objectives including: training and materials, donations, bundling of vaccines to improve delivery and injection equipment.
How have the companies responded? "The companies have exceeded our expectations," says Steve Jarrett, Deputy Director of the UNICEF Supply Division. "There are many interesting offers on the table."
"In the vaccine business, there are enough uncertainties during the development and manufacturing process; UNICEF is committed to working with industry to establish a reliable and predictable vaccine supply environment," says Jarrett.
|
Bulk orders
With the global increase in immunization coverage – from about 5% in 1974 to nearly 75% today, UNICEF’s purchasing role has grown; in 1999, UNICEF supply division shipped 104 million doses of measles vaccines, 102 million doses of BCG, 100.4 million doses of tetanus toxoid, and 90 million doses of combined diphtheria/tetanus/pertussis (DTP) vaccine.
Of course, with the increasing demands of the global polio eradication efforts, the bulk of UNICEF’s vaccine purchases in 1999 were for oral polio vaccine (OPV) – UNICEF shipped 881 million doses of OPV in 1999. |
Immunization Focus November 2000 - Contents
 |