|
||||
|
|
|||||
|
November 2000 Return to November 2000 contents page UPDATE Research that delivers results As GAVI decides on its R&D priorities, Karen Birmingham investigates neglected areas ASK anyone in public health to name some priorities for vaccine research in developing countries, and it’s a fairly safe bet that they will mention new vaccines against HIV, tuberculosis and malaria. But some will mention other equally pressing problems. One in four children worldwide is still not immunized routinely with existing, inexpensive vaccines. Up to half of all vaccinations given worldwide may be unsafe, putting children at risk of fatal bloodborne infections. And some important vaccines are stagnating in the development process because current, market-based systems offer manufacturers little incentive to produce them for developing countries. A growing number of specialists believe that these problems must be addressed, perhaps even before another dollar is spent on making new vaccines. "There are things we already know how to do, and for those we don’t need research, we need implementation," says Mark Kane, director of the Bill and Melinda Gates Children’s Vaccine Program. "But we also need to do some operational research to learn more and document the effectiveness of newer approaches and technologies." This month, the GAVI Board will begin to answer the question of how the partners in the Alliance, and the Vaccine Fund, should support R&D to accelerate the introduction of immunization products, systems and technologies that will benefit the world’s poorest. The Vaccine Fund will be one channel of support. The size of the budget for R&D has yet to be determined (see Box).
GAVI was formed to close the gaps in the world’s current immunization efforts, not to duplicate the efforts of others. It is therefore expected to support a few carefully targeted areas of R&D that are currently relatively neglected, rather than duplicate other funding sources. Some of these targeted areas may include operational research – for example, analyses of what incentives companies need to develop products that benefit mainly the poorest populations; measuring the outcomes of training health workers in safe practices; or measuring the burden of diseases in developing countries where data are scarce. Immunization Focus asked a number of major funding bodies in infectious disease research for information about the relative amounts they spend on basic, clinical and operational research. Not surprisingly, comparable data are not available, because research is categorized differently in different institutions. However, the institutions contacted largely agreed that operational research is underfunded. To define its priorities, GAVI will look at proposals from several sources, including its newly formed R&D Task Force. The Task Force, co-chaired by Myron Levine of the Center for Vaccine Development at the University of Maryland and a member of the GAVI Working Group, Yasuhiro Suzuki of WHO and Rino Rappuoli, of Chiron, has consulted widely to help clarify GAVI’s role. The group asked Peter Wilson, a consultant with more than 20 years’ experience of working with the pharmaceutical and vaccine industry, to canvas the opinion of a broad range of individuals with a stake in immunization. Wilson devised an 8-point questionnaire which asked respondents to prioritize aspects of R&D according to whether they are "central to, peripheral to or outside" the scope of the Task Force. The exercise identified three vaccines that are relatively near to market, but currently neglected, as strong candidates for support to overcome the final obstacles of development: pneumococcal conjugate vaccines that would protect against the strains of Streptococcus pneumoniae that are prevalent in developing countries; rotavirus vaccines; and meningococcal A vaccine. These three were selected because, as the Task Force puts it, they are "low-hanging fruit" that is almost ready to be plucked, and their potential benefit to public health is clear. The Task Force recognizes that malaria, HIV and TB vaccines are high priorities, but says that there is already a "massive global effort" devoted to them, and points out that the infrastructures for delivering them in developing countries are not yet in place. By devoting resources instead to the three near-ready vaccines, the Alliance could also help to prepare the infrastructures for delivering vaccines against malaria, HIV and TB when these become available, says the Task Force. In addition to the three products, the Task Force agreed to select up to three further projects. The direction that these will take is likely to emerge at the November meeting. Respondents to Wilson’s questionnaire also put a high priority on research to measure the burden of specific vaccine-preventable diseases in developing countries. Such data are valuable, not only to policy-makers but also for vaccine manufacturers, who increasingly rely on this information to calculate the potential market value of new vaccines. Disease burden data have been shown to be one of three key factors influencing take-up of hepatitis B and Haemophilus influenzae type b vaccines into national immunization programmes1. Orin Levine of the US National Institutes of Health (NIH), who organized a two-day meeting on disease burden at the WHO’s headquarters in Geneva in October, sums up the need for this data: "Simply put, countries are not going to consider paying for a vaccine to prevent a disease that they don’t think they have." However, Levine points out that for many diseases prevented by new vaccines, such as pneumonia caused by Hib and diarrhoea caused by rotavirus, establishing the local burden of disease is tricky. "Unlike measles or polio, there is no clinical disease entity that is unique to these agents. Carole Heilman, Director of the Division of Microbiology and Infectious Diseases at NIAID, whose organization is funding a trial in the Gambia of a 9-valent pneumococcus vaccine, also acknowledges the importance of this data. "The question high-burden countries have is, is this vaccine of use to them?" she says.
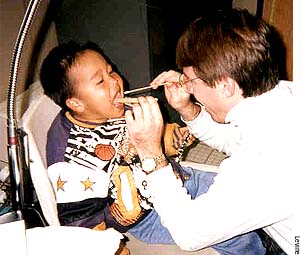
Say "aaah": Orin Levine investigates the burden of Hib in Alaska
Improving injection safety Spending on research to establish disease burden is probably very small at present, but figures are, once again, difficult to obtain. Heilman, for example, says she has hired a staff member specifically to work on the burden of Hib in the Gambia. However, she admits that she cannot give an estimate of how much money NIH invests in disease-burden research. Sometimes, when disease burden data are lacking, it falls to investigators to collect this as part of a clinical trial. Take, for example, the Phase III trial of an 11-valent pneumococcus vaccine in the Philippines. Principal investigator, Hanna Nohynek of the Finnish National Public Health Institute, says: "Because the figures on prevalence of pneumococcal disease aren’t available, we’ve built a disease burden component into the trial. On the basis of this, we should be able to calculate the savings of introducing the vaccine into such a community." Developing countries also need better methods to monitor vaccine coverage. Chile is frequently held up as a model of success for its immunization programme. "But our system for monitoring coverage is very primitive," says Rosanna Lagos of the Roberto del Rio Hospital in Santiago, and a member of the R&D Task Force. "Vaccination clinics have to resort to counting the number of doses at different ages – after a year or 6 months – to estimate the number of children vaccinated." Lagos says the programme desperately needs a computerized subject monitoring system. Another concern is unsafe injection techniques. Millions of injections are delivered each year in developing countries. As many as 50% of injections have been estimated to be unsafe in one study2. One model estimates that unsafe injection techniques may account for approximately 2.3-4.7 million hepatitis C infections, 80,000-160,000 HIV infections, and a staggering 20% of all new hepatitis B infections in developing countries3. Research to document the impact of using auto-disable syringes on reducing these infections is on the R&D Task Force agenda. Another area that the GAVI partners will be exploring is the need to document the impact of communication efforts such as public education and advocacy campaigns. Barry Bloom, dean of Harvard School of Public Health and a member of the R&D Task Force, points out that the diseases prevented by newer vaccines, such as hepatitis B and Hib, may not be well understood by people in developing countries. "The concept of a vaccine that can prevent liver cancer many years later is hard for people to grasp," he says. With only limited funds, the Alliance must be selective. But as key tasks are chosen, there is hope that each will bring the objective of safe universal immunization a little closer. Karen Birmingham is news editor of the journal Nature Medicine References 1. A model to estimate the probability of hepatitis B- and Haemophilus influenzae type b vaccine uptake into national vaccination programs. Miller MA, et al. Vaccine 2000 18: 2223-30. 2. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Simonsen, L. et al. Bulletin of the WHO. 1999; 77: 789-800. 3. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Kane A. et al. Bulletin of the WHO. 1999; 77: 801-7.
Return to November 2000 contents page
|
|||||
| Contact GAVI | Guestbook | Text version | Credits and Copyright
|
|||||